How to supplement iron in aquaponics. to supplement iron, chelated iron must be added to systems. admissible under usda organic standards, chelated iron is an artificially chelated iron ion—essentially, iron attached to an organic molecule to make it soluble. by adding chelated iron, iron deficiencies in your plants can be avoided.. Description. chelated iron – eddha-fe 6%. fully effective between ph levels in the high 5’s all the way up to the mid 9’s. this comes highly recommended by us. add directly to your system. you can apply iron at rate of 2mg/l every 2-3 weeks (1mg/l = 0.001g/l) apply foliarly (mixed with water and sprayed over plants).. Iron chelate helps to correct chlorosis (yellowing) in leaves caused by iron deficency in the aquaponics system. in an aquaponics system fish waste gets broken down by bacteria to create nitrates for plant growth. however, in many aquaponic systems there is often deficencies in other nutrients, in particular potassium, calcium and iron,.
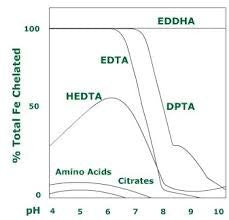
Chelation also occurs in mature aquaponic grow beds, but if you need to correct an iron deficiency through a quick hit of iron you need to supply your own chelated iron. there are different forms of chelated iron, each known by the agent used to perform the chelation. the most common are fe-edta, fe-dtpa, and fe-eddha.. In an aquaponic system, we have to rely on some sort of chelate to keep iron available to our plants. without a chelator, iron is only stable at ph levels below 4. fe-edta is the most common form of chelated iron found, but edta is only stable under acidic conditions and it’s breakdown products are phytotoxic.. For hydroponic and aquaponic applications the optimal iron concentrations of the water are 2.0 mg/l or 7.57 mg/gal. to measure your iron concentration try our hanna iron checker . * most plants have developed some means to assist with absorption of the insoluble ferric iron and some can even directly uptake it.






0 komentar:
Posting Komentar